Tweet Linkedin Whatsapp Electronic mail it Microbial limit test is performed to determine regardless of whether drug products comply with a longtime specification for microbial quality.
TYPES OF WATER You'll find many various grades of water utilized for pharmaceutical applications. Various are described in USP monographs that specify takes advantage of, acceptable methods of planning, and good quality attributes. These waters might be divided into two basic types: bulk waters, which are generally manufactured on web page exactly where They're used; and packaged waters, that happen to be generated, packaged, and sterilized to preserve microbial high-quality during their packaged shelf life.
Also, the absence of Preliminary positives could point out that the test has not been validated to display that there's no carryover of inhibition from the products or preservative.
Microbiologists in our Baltimore District are specialist on the usage of automatic microbic analytical methods. They ended up the initial FDA laboratory to implement these types of tools and possess substantial knowledge in validating methods for these pieces of apparatus.
A lot of the investigations/inspections of the recalled products began with a list of First sterility test failures. FDA assessment with the producer's output, controls, investigations and their inadequacies, coupled Together with the proof of product failure (Preliminary sterility test failure) in the end led to the motion.
Should the product or service being examined has antimicrobial action This is often thus far as feasible removed or neutralized.
As an example, it can be commonly identified that Pseudomonas cepacia is objectionable if present in a topical product or service or nasal Resolution in significant quantities; nevertheless, there are no test methods presented during the USP which will empower the identification with the existence of the microorganism.
Put together sample by dissolving 10g of products beneath test in one hundred ml of Soybean Casein digest medium.
), but for every monograph You can find an implied lessen limit down below which the desired solubilization outcome would not take place. Nonmonographed Analytical Waters Both Typical Notices and Requirements as well as introductory section to Reagents, Indicators, and Answers clearly point out that wherever the phrase “drinking water,” without the need of qualification or other specification, is indicated to be used in analyses, the caliber of h2o shall be Purified H2o. Nevertheless, several these qualifications do exist.
Almac Sciences’ revolutionary Digital tour solution digitises regular on-web-site customer tours and audits.
USP and USP for objectionable organisms are generally the recommendations for testing as specified by FDA prerequisites. Usually, the microbial limit test handles the next 3 test goods:
Examine administration's method to audit the caliber of the laboratory perform performed by outside the house contractors.
Microbial contamination can arise in various elements of drug production method like raw and auxiliary resources, drinking water, air, workshop gear and packaging components. To prevent air pollution, corresponding actions shall be formulated to improve sanitation administration to make certain environmental sanitation, material sanitation, method sanitation, plant sanitation and personnel sanitation in drug manufacturing.
A validation approach to get a water program usually incorporates the next actions: (1) creating standards for high-quality attributes in the completed water and also the supply water; (two) defining acceptable device read more operations and their operating parameters for acquiring the specified completed h2o high quality attributes with the out there resource drinking water; (3) selecting piping, equipment, controls, and monitoring technologies; (four) creating an IQ stage consisting of instrument calibrations, inspections to validate which the drawings precisely read more depict the final configuration in the h2o system and, exactly where needed, Specific tests to confirm that the set up satisfies the design specifications; (5) building an OQ phase consisting of tests and inspections to confirm which the machines, program alerts, and controls are working reliably and that proper warn and action levels are recognized (This stage of qualification may perhaps overlap with aspects of the following phase.
 Joshua Jackson Then & Now!
Joshua Jackson Then & Now!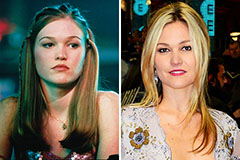 Julia Stiles Then & Now!
Julia Stiles Then & Now! Lisa Whelchel Then & Now!
Lisa Whelchel Then & Now! Erika Eleniak Then & Now!
Erika Eleniak Then & Now! Sarah Michelle Gellar Then & Now!
Sarah Michelle Gellar Then & Now!